NEWS CENTER
Research progress of self-healing coating materials
- Time of issue:2018-10-11 18:51
(Summary description)Self-repairing (Self-repairing) material is a branch of smart materials. It simulates the self-repairing mechanism of biological damage and self-repairs the damage caused by the material during use.
Research progress of self-healing coating materials
(Summary description)Self-repairing (Self-repairing) material is a branch of smart materials. It simulates the self-repairing mechanism of biological damage and self-repairs the damage caused by the material during use.
- Categories:Industry News
- Author:
- Origin:Northeast Petroleum University
- Time of issue:2018-10-11 18:51
- Views:
Self-repairing material is a branch of smart materials. It simulates the self-repair mechanism of biological damage and self-repairs the damage caused by the material during use. Among many self-healing materials, the research and development of self-healing coatings that can protect the substrate and impart special properties to the substrate has become a hot spot in the scientific community. It is used in conductive coatings, anti-corrosion coatings, scratch-resistant coatings and other fields. It has a wide range of applications, especially in some high-end areas with harsh conditions and difficult to maintain, such as special adhesive coatings used in aerospace and military marine applications, marine drilling platforms and underground oil pipelines and other anti-corrosion coatings. Urgent needs.
At present, self-healing coatings are divided into foreign aid self-healing coatings and intrinsic self-healing coatings according to the type of repair. Foreign aid type self-healing coating refers to the self-repairing function of the coating matrix by introducing additional components such as microcapsules, carbon nanotubes, microvessels, glass fibers or nanoparticles containing a repair agent system. This method requires Various repair agent systems are pre-embedded and then added to the matrix. When the material is damaged, the repair agent in the damaged area will be released under the action of external stimuli (force, pH value, temperature, etc.), thereby achieving self-repair. Intrinsic self-repair does not require an external repair system, but the coating material itself contains special chemical bonds or other physical and chemical properties such as reversible covalent bonds, non-covalent bonds, molecular diffusion, etc. to achieve self-repair functions. This method does not rely on the repair agent, eliminates the need for complex steps such as pre-repair agent embedding technology, and has little effect on the performance of the substrate. However, the molecular structure design of the coating substrate material is the biggest challenge faced by the method, and it has become a research focus. .
This article summarizes the latest research progress in the field of self-healing coatings in recent years, focusing on reviewing the types, mechanisms and applications of foreign-aid self-healing coatings and intrinsic self-healing coating systems, and prospecting the application prospects of self-healing coatings.
1. Foreign aid type self-healing coating
1.1 micro/nanocapsule filling type self-healing coating
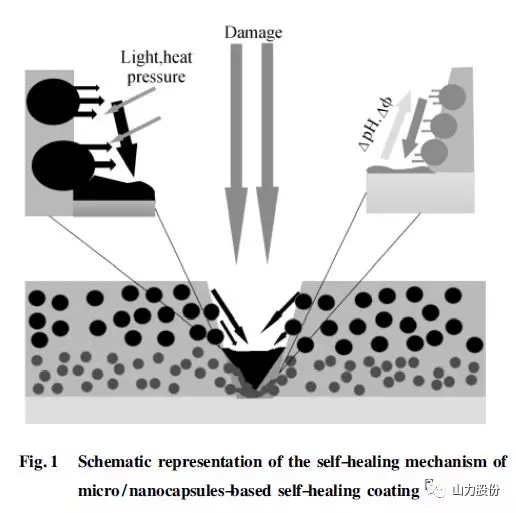
The self-repairing method of microcapsules is currently the most used method in the field of self-repairing coatings. Since White et al. first reported on the self-repairing mechanism of microcapsules in 2001, it has recently received extensive attention from scientific researchers. The self-healing mechanism of the micro/nanocapsule-filled self-healing coating is shown in Fig.1. The micro/nanocapsule containing the repairing agent is pre-embedded in the polymer matrix or coating. When the matrix or coating material is damaged When (initiated by light, heat, pressure, pH changes, etc.), the capsule ruptures and releases the repairing agent. When the repairing agent encounters the catalyst in the substrate or coating, a cross-linking curing reaction occurs to repair the cracked surface and realize the self-repair of the damaged part. . At present, this method has been widely used in the field of coating materials.
1.1.1 Encapsulated corrosion inhibitor system: the corrosion inhibitor is microencapsulated and then used as a self-healing coating, which is mainly used in the field of metal anti-corrosion coatings. This method avoids the high toxicity and destabilization of the coating due to corrosion inhibition It is not suitable to directly add the shortcomings in the coating. Kumar et al. and Mehta et al. prepared microcapsules containing different types of corrosion inhibitors, discussed the influence of the particle size of the microcapsules on the stability of several different coating systems, and studied the release ability of corrosion inhibitors when the microcapsules were broken. The coating containing corrosion inhibitor microcapsules is applied to the steel plate, showing a good anti-corrosion effect. Zheludkevich et al. reported an environmentally friendly microcapsule with chitosan as the wall material and green corrosion inhibitor cerium ion as the core material. pH changes in a corrosive environment cause the release of cerium ions to achieve the anti-corrosion performance of the coating. Koh et al. prepared polyurethane-encapsulated microcapsules of isosorbide derivative corrosion inhibitors. Tests show that the coatings containing microcapsules have good anticorrosive and self-repairing functions. Sauvant et al. proposed a self-healing mechanism of inorganic film-forming corrosion inhibitors. Microcapsules with a particle size of 10 to 240 μm were prepared with MgSO4 as the core material, embedded in the coating material and coated on the surface of the steel. When corrosion occurs When the microcapsules are broken, the released Mg2+ will automatically migrate to the crack under the action of the anode, and deposit Mg(OH)2 under the action of a certain pH to seal the crack.
1.1.2 Encapsulated dry oil system: The preparation of self-healing coatings with dry oil as a repairing agent is also the main trend in current research. The mechanism is that the dry oil released after the microcapsule ruptures is oxidized by oxygen after contact with air. For the self-healing film, linseed oil and tung oil are the most commonly used drying oils. Suryanarayana et al., Behzadnasab et al., Karan et al., Szabó et al., Majdeh et al. prepared micron-sized (20~150μm) urea-formaldehyde resin microcapsules containing linseed oil and linseed oil/CeO2. They respectively investigated the preparation process parameters such as stirring rate, The effect of reaction time on the formation of capsules, and the influence of the amount of microcapsules added on the mechanical properties of the coating was investigated. The experimental results show that the microcapsules have sufficient strength and can withstand a certain amount of shear during the preparation and spraying of the adhesive coating. The microcapsule has a rough surface, which facilitates good bonding with the bonding coating and the substrate interface; when the coating cracks, the microcapsule breaks and releases the repairing agent, which has good self-repair and anti-corrosion properties.
Masoumeh et al. added micro/nanocapsules containing linseed oil to the epoxy resin coating material. The smallest capsule size is 450nm and the largest is 6μm. The study pointed out that the addition of microcapsules at room temperature makes the coating material strong. And the flexibility is slightly reduced, and the flexibility is greatly reduced at high temperatures, and the coating exhibits good self-repairing properties to the metal. Eshaghi et al. prepared silane coupling agent-modified vinyl cellulose coated linseed oil microcapsules with a particle size of 5~35μm. The focus was on the grafting efficiency of silane coupling agent and vinyl cellulose. The presence of the linking agent will make the microcapsule and the water-based acrylic resin coating substrate have good interfacial adhesion properties. Zhao Peng et al. prepared microcapsules with a particle size of 1-50μm using tung oil as the core material, applied them to a 150μm thick coating and applied it to the surface of tinplate, and observed that the coating has good self-repair and Anti-corrosion performance.
1.1.3 Encapsulated reactive repair agent system: The repair agents such as dicyclopentadiene (DCPD), epoxy resin, silicone series reagents and reagents with special functional groups are encapsulated in microcapsules. These reagents have certain characteristics. After being released from the microcapsules, it will be polymerized to form a cross-linked structure when it is released from the microcapsules when it comes in contact with the catalyst or is triggered by ultraviolet light, heat, oxygen, etc., so as to bond the cracks and realize self-healing. Among them, epoxy resin is used as a self-healing agent in many reports. For example, Liu et al. added microcapsules with epoxy resin as the repairing agent to the epoxy coating. The coating adopts an amide curing agent. On the one hand, the coating resin is cured. On the other hand, the excessive amide can polymerize with the epoxy resin repairing agent released by the ruptured microcapsules to realize the self-repairing function. The coating material has good self-repairing performance and good anti-corrosion effect on carbon steel. Liao et al. prepared epoxy resin self-healing coatings with urea-formaldehyde resin coated E-51 epoxy resin microcapsules as the repair system and also showed good self-healing effects. Self-healing coatings containing silicone repair agent microcapsules have also been reported. Using the reactivity of the vinyl on the molecular chain of the repairing agent, adding some photosensitizers, the repairing agent overflows when the microcapsules are destroyed under the action of external force, and under the action of ultraviolet light radiation The repair agent can react quickly to realize the self-repair of the coating. Song et al. prepared microcapsules containing polydimethylsiloxane repair agents with functional ends. The system can initiate polymerization under ultraviolet light or sunlight to achieve self-repair. It is environmentally friendly and can be achieved multiple times by photoinitiation. Self-healing, this is the first reported capsule type reproducible self-healing system. Huang et al. [19, 20] prepared microcapsules with perfluorooctyltriethoxysilane as the repairing agent. The particle size of the capsules is 40~400μm. Repair performance, and has good anti-corrosion performance on steel, its self-repair mechanism is realized by forming a network structure after the repairing agent is hydrolyzed. In addition, they also prepared polyurethane (PU) coated microcapsules of hexamethylene diisocyanate, and discussed the influence of the particle size and content of the microcapsules on the self-healing properties of the coating. They concluded that the particle size of the microcapsules should not be less than 100μm. , The coating has good self-repair and anti-corrosion effects when the mass fraction of microcapsules is not less than 5%.
1.2 micro/nano container filling type self-healing coating
There are many reports on the use of hollow micro-nano spheres or mesoporous micro spheres and other micro-nano containers to load corrosion inhibitors in the field of self-healing anti-corrosion coatings.
For example, a layer-by-layer assembly method is adopted, with nano-SiO2, kaolin or porous nano-TiO2 particles as the core, and the outer layer is deposited with a multi-layer polyelectrolyte nano-active unit containing corrosion inhibitor benzotriazole (BTA) to prepare a metal anticorrosive coating. When corrosion occurs, changes in pH (chemical corrosion processes are mostly accompanied by changes in pH) cause changes in the structure and permeability of the polyelectrolyte layer of the active unit, releasing corrosion inhibitors, forming an adsorption layer on the metal surface, and passivating the metal surface , Effectively prevent metal corrosion. Fu et al. prepared SiO2 microspheres loaded with antiseptic caffeine molecules, and modified pH-sensitive cucurbituril ferrocene on their surface to achieve controllable release of corrosives under different acid-base conditions, and applied them to Among the anti-corrosion coatings on the aluminum alloy surface, it has a good self-repairing effect. Zhao et al. prepared hollow raspberry polystyrene submicron spheres with open pores on the surface. The microspheres are loaded with corrosion inhibitor BTA. The surface pores of the microcapsules are opened under acid-base conditions and closed under neutral conditions. The controllable release of BTA is realized. The submicron capsules are applied to polyurethane anticorrosive coating and applied to copper metal surface to show good anticorrosive function. Li et al. prepared a silicon/polymer double-wall hybrid nanotube container, with porous silicon as the inner wall of the container and a polymer layer as the outer wall. Different polymer outer layers can be selected to achieve the controllable release of the core material. The silicon/polymer double-walled nanocontainer with value sensitivity, temperature sensitivity and redox responsiveness is loaded with a corrosion inhibitor benzotriazole in the nanotube container, and the self-healing coating exhibits good self-repairing function. Rahimi et al. [26] prepared a silicone nanocontainer containing a mixture of 2-mercaptobenzothiazole (MBT) or 2-mercaptobenzimidazole (MBI) corrosion inhibitors and α-cyclodextrin (α-CD) , MBT or MBI and α-CD can form hydrogen bonds when encountering a humid environment, thereby playing a self-repairing effect. The nano-container is applied to aluminum surface coating to study its anti-corrosion and self-repairing properties, and the effect is obvious NS.
Borisova et al. used mesoporous silica as a container with a corrosion inhibitor loaded in the container, and investigated the influence of the size of the nano-container on the self-healing performance of the coating. Recently, Chen et al. reported a mesoporous silica nanocontainer that can be controlled by ultraviolet light. The container is filled with corrosion inhibitor benzotriazole. The mesoporous structure of the silica surface can be introduced by introducing azobenzene functional groups. The chemical structure of the mesopores can be changed under the irradiation of ultraviolet light to realize the opening and closing of the mesopores. In this way, not only the release of the antiseptic can be controlled, but also the multiple self-repairing of the coating can be realized.
1.3 shape memory fiber yarn/polymer self-healing coating
shape memory fiber is a metal alloy or polymer with a shape memory effect. After the material is deformed under the action of external force, it can be heated to a certain temperature to restore its original shape. For example, shape memory polymer fibers and thermoplastic particles are embedded in an epoxy resin material, the shape memory fibers are used as the skeleton structure of the self-repair system, and the thermoplastic resin is used as a repair agent. When the material cracks, the damage is heated to above the glass transition temperature of the shape memory fiber. The pre-stretched fiber will shrink due to the shape memory effect. The shrinkage force pulls the matrix material to close the crack. At the same time, When the thermoplastic resin particles are heated to the melting temperature, they begin to flow, fill the cracks, and finally realize self-healing. The Leng Jinsong research group of Harbin Institute of Technology has also studied a large number of shape memory polymers. Among them, shape memory epoxy polymer (SMEP) is used as the matrix and thermoplastic polycaprolactone (PCL) is used as a repair agent to prepare a self-repairing polymer. Functional shape-memory polymer. This type of polymer can repair the damaged area for 3 cycles, with a maximum repair efficiency of 67.87%, which has great application value.
2. Intrinsic self-healing coating
Intrinsic self-healing coating refers to the coating material itself containing special chemical bonds or functional groups, which after damage occurs through the reorganization of chemical bonds, the reaction of functional groups or physical effects to achieve self-repair. Compared with foreign aid-type self-healing coatings, this method does not have any added substances such as microcapsules, microcontainers, etc., so it will not have a major impact on the mechanical properties of the coating material matrix, but it does not affect the coating matrix material. Therefore, the difficulty of preparation is higher than that of foreign aid self-repairing system.
2.1 UV light induced self-healing coating
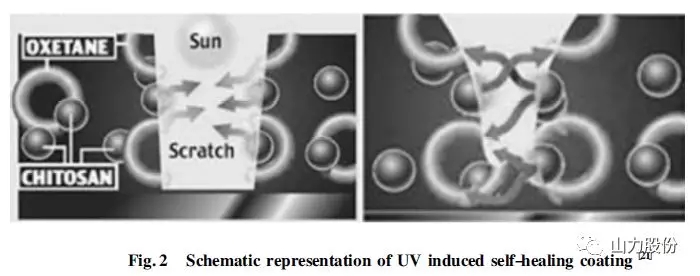
Ghosh et al. prepared a polyurethane coating with self-healing function. The self-healing mechanism is shown in Fig.2. The polyurethane network structure in the coating contains chitosan and oxetane structures. When the surface of the coating is scratched, the oxetane ring structure is broken, exposing two ends that can produce chemical reactions. When irradiated by ultraviolet light, the exposed ends of chitosan and oxetane in the coating attract each other and combine to repair the ring structure, thereby realizing the self-repair of the damage of the coating.
Supramolecular polymer is a kind of material that can realize self-repair function under the action of ultraviolet light. Coulibaly et al. prepared a supramolecular polymer. This material is formed by chelating a short-chain polymer with a telechelic structure and a metal ligand (zinc or lanthanum). The metal ligand is polymerized with low molecular weight and high molecular weight. The objects are connected by non-covalent bonds (ionic bonds). When irradiated by ultraviolet light, the energy absorbed by the metal ligand is converted into heat, the non-covalent bond is broken, and the metal ligand will temporarily detach from the polymer, so the relative molecular weight of the polymer decreases, the viscosity decreases, and it becomes a flowable state. When the material is cracked or damaged, after UV light is applied to the damaged area, the molecules in the flowable state can fill the damaged area to realize self-repair. In the experiment, a scratch with a depth of 200 m was made on a 400 m thick plastic coating. After irradiating 2 times under ultraviolet light, after 30s each time, the scratches can be repaired well, and the repairing efficiency can reach 100%±36%.
Wang et al. developed polydimethylsiloxane-polyurethane (PDMS-PUR) and polyethylene glycol-polyurethane (PEG-PUR) network structures with CuCl2 as a catalyst and UV-induced self-healing function. Self-repair is achieved by the reorganization and conformational changes of supramolecular or covalent bonds triggered by ultraviolet light irradiation.
2.2 Thermally reversible cross-linked self-healing coating
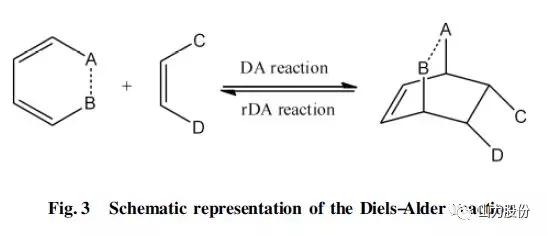
The thermally reversible cross-linking self-healing coating mainly relies on the characteristic functional group substances that can undergo Diels-Alder reversible cross-linking reaction in the coating matrix, and realizes the self-healing of the coating through the DA reversible reaction. The DA reversible reaction mechanism is shown in Fig.3 . Wouters et al. prepared a copolymer of furan methacrylate (FMA) and butyl methacrylate (BMA) by free-radical copolymerization. The functionality (hardness, elastic modulus) of the copolymer can be adjusted by adjusting the ratio of FMA and BMA. , Crosslinking density) and glass transition temperature, use this copolymer and bismarimide to polymerize to prepare a powder, apply the powder to the aluminum surface to prepare a self-healing powder coating, and heat the powder coating to 175°C The polymer film is formed and used when it is cooled to room temperature. When the coating is scratched and damaged, the polymer film is reheated to 175°C, the film reflows, and the damaged area can be repaired in 30 seconds. It can be repaired multiple times without affecting the performance of the matrix. Pratama et al. prepared a self-healing thermosetting resin coating based on DA reaction. They microencapsulated maleimide, a monomer that can react with DA, and introduced difuran, another monomer that can react with DA, into the coating. Furan functionalized epoxy resin coating is prepared in the substrate. Experimental results show that the self-healing efficiency of the coating with 10% microcapsules with a particle size of 185 μm can reach 71%. Postiglione et al. prepared a trifunctional and difunctional furan resin and bismaleimide self-healing coating system. The system has DA reaction at 50°C and reverse DA reaction at 120°C, and passes through the coating matrix Adding plasticizer benzyl alcohol to improve the self-healing performance, the experimental results show that the coating system can achieve 48% mechanical strength recovery.
2.3 Self-healing polymer membrane assembled layer by layer
layer-by-layer assembly self-healing polymer film is based on the action of intermolecular non-covalent forces. It constructs a composite coating film through reciprocating interface assembly, and introduces various types of functional groups into the coating film to control the mechanical properties and self-repair properties of the coating film. Andreeva et al. prepared a layer-by-layer assembly self-healing film containing a repair agent. They assembled the anticorrosion agent 8-hydroxyquinoline into the polymer film layer. When the coating was damaged, it passed through the movement and resistance of the polymer chain. The overflow of the corrosive realizes the self-repair of the assembled polymer film layer by layer, and has good corrosion resistance. Sun Junqi’s research group at Jilin University used an exponentially growing layer-by-layer self-assembly method to construct a branched polyvinylimine (bPEI)/polyacrylic acid (PAA) polyelectrolyte coating. Self-repair is completed within 10s. Self-repair can be achieved by immersing the coating in water or spraying water on the scratched surface. At the same time, the (bPEI/PAA)*30 film can be self-repairing multiple scratches on the same position. The self-repair mechanism is that during the film preparation process, the polymer chains in the bPEI/PAA film can be controlled to intersperse in the film. The prepared film is stable in the air, while the polymer chains can be Flowing or swelling occurs to repair the damaged area. Hu Xiaoxia and others prepared a polyurethane/sodium carboxymethylcellulose (PU/CMC) multilayer film by layer-by-layer assembly technology, which has self-repairing ability. They also introduced a third polyelectrolyte polydimethyldiallylammonium chloride (PDDA) into the membrane structure. The prepared PDDA(CMC/PU)n membrane showed an enhanced self-repairing effect. Within a few seconds of immersion in salt water, scratches with a width of 20 to 30 μm can be self-repaired.
3. Conclusion
The research in the field of self-healing coatings has been developed by leaps and bounds in the past 10 years. The current and future research focuses on the optimization of the original self-healing system, the discovery of new self-healing mechanisms, and the design of recyclable self-healing materials. And the construction and application of self-healing coating materials. The research in this field involves chemistry, materials science, mechanics and other multidisciplinary cross-discipline, which requires more scientific research enthusiasts to invest in it. I believe that self-healing technology will have broad application prospects.
Hot News

黄石山力科技股份有限公司
Sunny Technologies Incorporation Limted

Contact us
Add:No. 2, Guangzhou road, tuanshancheng Development Zone, Huangshi City, No. 101, Gaoxin 6th Road, Donghu New Technology Development Zone, Wuhan
Mail:spt@sunnychina.com.cn
Tel:156 7177 7755 / 027-59715061
Fax:027-59715060





